References and regulatory statements
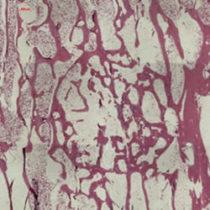
1. Yang, H.L., et al., Bone healing response to a synthetic calcium sulfate/beta-tricalcium phosphate graft material in a sheep vertebral body defect model. J Biomed Mater Res B Appl Biomater, 2012. 100B(7): p. 1911-21. 2. Data on file, Mr Nissar & Mr Gopal. 3. Cooper, J.J., J.A. Hunt, and F. Pu, Enhancing the Osteogenic Potential of Bioabsorbable Implants through Control of Surface Charge., in Society for Biomaterials 2007 Annual Meeting. 2007: Chicago, Illinois, USA. 4. Data on file, Mr HK Sharma. 5. Pina, S. and J.M.F. Ferreira, Bioresorbable Plates and Screws for Clinical Applications: A Review. Journal of Healthcare Engineering, 2012. 3(2)
For indications, contraindications, warnings and precautions see Instructions for Use.
©2019, Biocomposites, genex and Power to Restore are trademarks/registered trademarks of Biocomposites Ltd. All rights reserved. No unauthorised copying, reproduction, distributing or re-publication is allowed unless prior written permission is granted by the owner, Biocomposites Ltd.
Patents granted: EP 1390086 B1, US 8632796, CN ZL02809194.9, US 8496955
MA0097R2