Clinical particulars
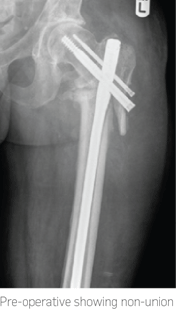
35-year-old male involved in a road traffic accident, suffered multiple injuries and subtrochanteric fracture of left femur. This was nailed, however subsequently, he developed infection and drainage from both at proximal and distal locking screw area. He went to theatre multiple times and developed wound approx. 15cm on the proximal lateral thigh, which was treated with VAC.
He presented a year later with discharging wound proximally and distally.
Treatment
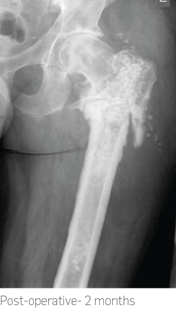
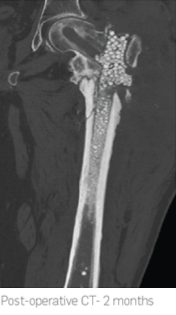
The femoral nail was removed followed by reaming of the femoral canal and wash-out procedure. 40cc of STIMULAN was used to carry the antibiotic, fill resulting dead space in the intramedullary canal and treat the bacterial infection. Cultures revealed infection to be
Staphylococcus aureus.
Outcome
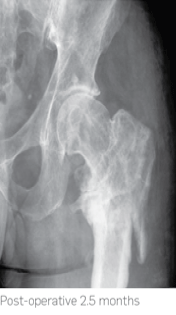
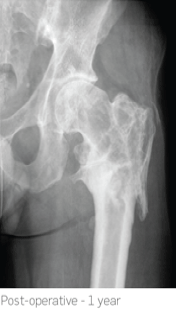
2.5 months post-operatively x-rays showed almost complete absorption of the STIMULAN beads and at 7 months there was complete healing of the non-union.
At 1 year follow-up the patient remains infection free, walking with no pain.
Note: See STIMULAN page for regulatory statements
.
MA0027R3




